Background: Pts with LR-MDS receiving luspatercept in the phase 3 MEDALIST trial previously reported durable, long-term red blood cell (RBC) transfusion independence (RBC-TI) (Fenaux P, et al. J Clin Oncol 2022;40[suppl 16]. Abstract 7056). MEDALIST pts were eligible to enroll in a follow-up study to evaluate long-term efficacy and safety.
Aims: To report long-term efficacy and safety outcomes in ESA-refractory/intolerant pts with LR-MDS treated with luspatercept in the MEDALIST trial with an additional 26 mo of follow-up from the final MEDALIST data cutoff.
Methods: Eligible pts were ≥ 18 y of age; IPSS-R-defined Very low-, Low-, or Intermediate-risk MDS with ring sideroblasts; were refractory, intolerant, or unlikely to respond to ESAs, and required regular RBC transfusions. Pts were randomized 2:1 to luspatercept (starting dose 1.0 mg/kg, with titration up to 1.75 mg/kg) or placebo. Efficacy outcomes include RBC-TI ≥ 8 wk and ≥ 16 wk. Cumulative duration of response was determined by Kaplan-Meier (KM) analysis. Rates of longest single and cumulative duration of RBC-TI ≥ 1 y and exposure-adjusted incidence rates (EAIR) of treatment-emergent adverse events (TEAEs) and AEs of special interest (AESIs) were calculated. Rates of high-risk (HR)-MDS and acute myeloid leukemia (AML) progression were calculated from LR-MDS diagnosis to HR-MDS/AML diagnosis, or to last HR-MDS/AML follow-up date for pts who did not progress.
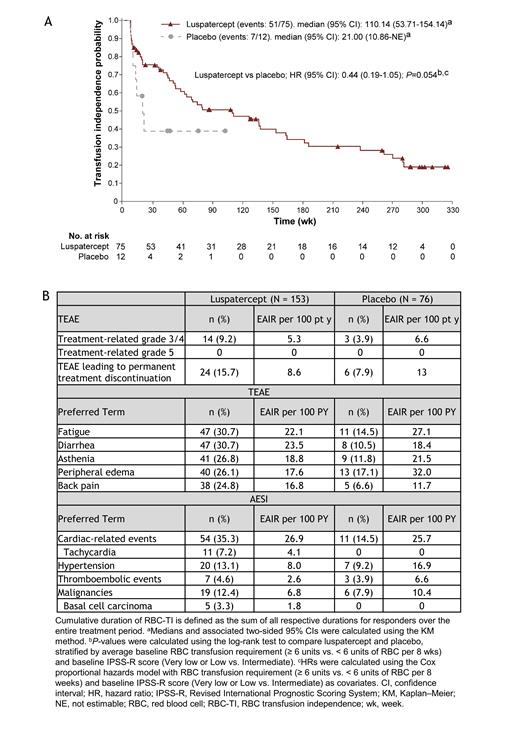
Results: As of January 2, 2023, 11 pts remained on luspatercept treatment; 8 pts randomized to luspatercept and none from placebo continued in post-treatment follow-up. The median (range) follow-up time was 39.9 (2.8-76.0) mo for luspatercept arm pts and 38.7 (1.7-68.6) mo for placebo arm pts. The median (range) duration of treatment was 50.9 (5.9-332.9) wk for luspatercept pts and 24.0 (9.0-103.0) wk for placebo pts. Seventy-five of 153 (49.0%) luspatercept pts achieved RBC-TI ≥ 8 wk during the entire treatment period and 53/75 (70.7%) had ≥ 2 response periods with a median cumulative duration of 110.14 wk (95% confidence interval [CI], 53.71-154.14; Figure A). Overall, 31/75 (41.3%) luspatercept pts had the longest RBC-TI duration ≥ 1 y, and 44/75 (58.7%) had a cumulative RBC-TI duration ≥ 1 y. In total, of the 48/153 (31.4%) pts receiving luspatercept who achieved RBC-TI ≥ 16 wk during the entire treatment period, 32/48 (66.7%) had ≥ 2 response periods with a median cumulative duration of 129.29 wk (95% CI, 79.86-240.43). Grade 3/4 treatment-related TEAEs were reported in 14/153 (9.2%; EAIR, 5.3 per 100 pt-y [PYs]) and 3/76 (3.9%; EAIR, 6.6 per 100 PYs) luspatercept and placebo pts, respectively; TEAEs of any grade leading to permanent treatment discontinuation were reported in 24/153 (15.7%; EAIR, 8.6 per 100 PYs) luspatercept and 6/76 placebo pts (7.9%; EAIR, 13.0 per 100 PYs). The most common TEAEs of any grade reported in > 5% of luspatercept pts were fatigue (47/153 [30.7%]; EAIR, 22.1 per 100 PYs; vs placebo, 11/76 [14.5%]; EAIR, 27.1 per 100 PYs), diarrhea (47/153 [30.7%]; EAIR, 23.5 per 100 PYs; vs placebo, 8/76 [10.5%]; EAIR, 18.4 per 100 PYs), asthenia (41/153 [26.8%]; EAIR, 18.8 per 100 PYs; vs placebo, 9/76 [11.8%]; EAIR, 21.5 per 100 PYs), peripheral edema (40/153 [26.1%], EAIR, 17.6 per 100 PYs; vs placebo, 13/76 [17.1%]; EAIR, 32.0 per 100 PYs), and back pain (38/154 [24.7%]; EAIR, 16.8 per 100 PYs; vs placebo, 5/76 [6.6%]; EAIR, 11.7 per 100 PYs; Figure B). During the entire treatment period, 26/44 (59.1%), 9/23 (39.1%), and 12/86 (14.0%) pts who received ≤ 1 mg/kg, 1.33 mg/kg, and 1.75 mg/kg luspatercept, respectively, reported fatigue of any grade. No new progression to HR-MDS or AML events have been reported since the last data cutoff (Santini V et al. Blood 2022;140[suppl 1]:4079-4081).
Conclusions: Pts with LR-MDS who received luspatercept for > 2 years longer than in the original MEDALIST study, continued to experience sustained periods of RBC-TI with more than half of pts experiencing cumulative RBC-TI for ≥ 1 year. The long-term safety profile of luspatercept is consistent with previous shorter-term reports, and AEs were mostly lower grade with rates of fatigue decreasing with increasing luspatercept dose. These data demonstrate the long-term efficacy and safety of luspatercept in pts with LR-MDS intolerant/ineligible for ESA treatment.
Disclosures
Santini:Janssen: Other: Travel support; Syros: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Komrokji:Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Consultancy; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Buckstein:Abbvie: Honoraria; Taiho: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Oliva:Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Grande Ospedale Metropolitano BMM: Current Employment; Daiichi: Consultancy, Honoraria; Ryvu: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Alexion: Consultancy, Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Sobi: Honoraria, Speakers Bureau; Servier: Patents & Royalties. Keeperman:Celgene: Divested equity in a private or publicly-traded company in the past 24 months; Pfizer: Divested equity in a private or publicly-traded company in the past 24 months; Bristol Myers Squibb: Current Employment. Rose:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Celgene: Current equity holder in private company. Giuseppi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Vilmont:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Lai:Bristol Myers Squibb: Current Employment. Miteva:Bristol Myers Squibb: Current Employment. Aggarwal:Bristol Myers Squibb: Current Employment. Platzbecker:Syros: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Curis: Consultancy, Research Funding; Janssen Biotech: Consultancy, Research Funding; Merck: Research Funding; Geron: Consultancy, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Honoraria; AbbVie: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Fibrogen: Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding. Fenaux:Janssen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; French MDS Group: Honoraria; Jazz: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Zeidan:Astellas: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Agios: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Astex: Research Funding; Orum: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Shattuck Labs: Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal